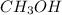Rank the compounds CH3OH CH3CH2OH CH3CH2CH2OH in terms of increasing evaporation rate. 1. CH3CH2CH2OH < CH3CH2OH < CH3OH 2. CH3OH < CH3CH2OH < CH3CH2CH2OH 3. CH3CH2CH2OH < Rank the compoundsCH3OH CH3CH2OH CH3CH2CH2OHin terms of increasing evaporation rate.1.CH3CH2CH2OH < CH3OH < CH3CH2OH2.CH3OH < CH3CH2CH2OH < CH3CH2OH3.CH3OH < CH3CH2OH < CH3CH2CH2OH4.CH3CH2OH < CH3CH2CH2OH < CH3OH5.CH3CH2CH2OH < CH3CH2OH < CH3OH

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1

Chemistry, 23.06.2019 09:00
Chortling is used to clean water. another possible atom that would also work is a. sodium b. sulfur c. bromine
Answers: 1

Chemistry, 23.06.2019 10:40
Question 17 hydrogen is manufactured on an industrial scale by this sequence of reactions: +ch4gh2og ⇌ +cog3h2g k1 +cogh2og ⇌ +co2gh2g k2 the net reaction is: +ch4g2h2og ⇌ +co2g4h2g k write an equation that gives the overall equilibrium constant k in terms of the equilibrium constants k1 and k2. if you need to include any physical constants, be sure you use their standard symbols, which you'll find in the aleks calculator.
Answers: 2

Chemistry, 23.06.2019 13:30
What would happen if no were added to n(g)+o2=2no(g) at equilibrium?
Answers: 1
You know the right answer?
Rank the compounds CH3OH CH3CH2OH CH3CH2CH2OH in terms of increasing evaporation rate. 1. CH3CH2CH2O...
Questions

Chemistry, 05.10.2020 15:01

English, 05.10.2020 15:01

Mathematics, 05.10.2020 15:01

Mathematics, 05.10.2020 15:01



Mathematics, 05.10.2020 15:01




Mathematics, 05.10.2020 15:01


Mathematics, 05.10.2020 15:01

English, 05.10.2020 15:01

Mathematics, 05.10.2020 15:01




Mathematics, 05.10.2020 15:01

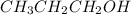 < CH_{3}CH_{2}OH[/tex] <
< CH_{3}CH_{2}OH[/tex] <