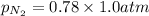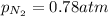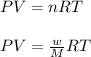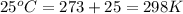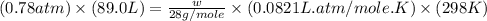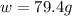Chemistry, 10.03.2020 01:16 andrew2786
Calculate the mass of nitrogen dissolved at room temperature in an 89.0 LL home aquarium. Assume a total pressure of 1.0 atmatm and a mole fraction for nitrogen of 0.78.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 23.06.2019 04:50
The diagin dilutepage 6 of 12a6a5(a)fluorine, chlorine, bromine and iodine are placed in the same group of theperiodic table.state the common name used to describe elements in this group.(i)state the group in which the elements are placed and explain whythey are placed in that group.(ii)which of the above named elements is a solid at roomtemperature and pressure?
Answers: 2

Chemistry, 23.06.2019 07:00
Determine the length of the object shown. 97.8 mm 97.80 mm 97 mm 98 mm
Answers: 1
You know the right answer?
Calculate the mass of nitrogen dissolved at room temperature in an 89.0 LL home aquarium. Assume a t...
Questions

Business, 30.03.2021 05:10

English, 30.03.2021 05:10

Mathematics, 30.03.2021 05:10

Mathematics, 30.03.2021 05:10




Mathematics, 30.03.2021 05:10


Social Studies, 30.03.2021 05:10




Mathematics, 30.03.2021 05:10

Chemistry, 30.03.2021 05:10

Chemistry, 30.03.2021 05:10



Mathematics, 30.03.2021 05:10

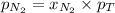
 = partial vapor pressure of nitrogen = ?
= partial vapor pressure of nitrogen = ?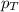 = total pressure = 1.0 atm
= total pressure = 1.0 atm = mole fraction of nitrogen = 0.78
= mole fraction of nitrogen = 0.78