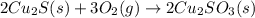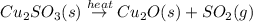Chemistry, 10.03.2020 00:40 leysirivera23ovez6n
When copper sulfide is partially roasted in air (reaction with o2), copper sulfite is formed first. subsequently, upon heating, the copper sulfite thermally decomposes to copper oxide and sulfur dioxide. Write balanced chemical equations for these two reactions

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1

Chemistry, 23.06.2019 03:30
Select the correct lewis structure for fluorine which is group 7a element?
Answers: 1

You know the right answer?
When copper sulfide is partially roasted in air (reaction with o2), copper sulfite is formed first....
Questions

Mathematics, 09.09.2019 04:20

Mathematics, 09.09.2019 04:20

Mathematics, 09.09.2019 04:20

Mathematics, 09.09.2019 04:20

Geography, 09.09.2019 04:20

Mathematics, 09.09.2019 04:20

Advanced Placement (AP), 09.09.2019 04:20

Biology, 09.09.2019 04:20


Mathematics, 09.09.2019 04:20


Mathematics, 09.09.2019 04:20




Mathematics, 09.09.2019 04:20

Mathematics, 09.09.2019 04:20

Mathematics, 09.09.2019 04:20

Biology, 09.09.2019 04:20