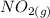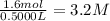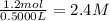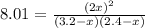Chemistry, 10.03.2020 00:27 TabbyKun00
Suppose a 500.mL flask is filled with 1.6mol of NO3 and 1.2mol of NO2 . The following reaction becomes possible: +NO3gNOg 2NO2g The equilibrium constant K for this reaction is 8.01 at the temperature of the flask. Calculate the equilibrium molarity of NO3 . Round your answer to two decimal places.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

You know the right answer?
Suppose a 500.mL flask is filled with 1.6mol of NO3 and 1.2mol of NO2 . The following reaction becom...
Questions






History, 16.08.2020 01:01

Mathematics, 16.08.2020 01:01


Mathematics, 16.08.2020 01:01




English, 16.08.2020 01:01


Mathematics, 16.08.2020 01:01

Social Studies, 16.08.2020 01:01




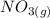 is 1.60 M
is 1.60 M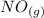 ⇄2
⇄2