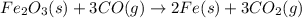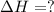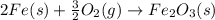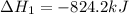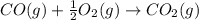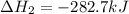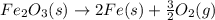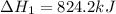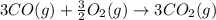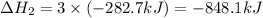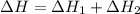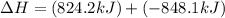Chemistry, 10.03.2020 00:25 skylar7192
Calculate ΔHrxn for the following reaction: Fe2O3(s)+3CO(g)→2Fe(s)+3CO2(g) Use the following reactions and given ΔH values: 2Fe(s)+32O2(g)→Fe2O3(s),ΔH CO(g)+12O2(g)→CO2(g),ΔH==−824.2kJ−2 82.7kJ

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 23.06.2019 00:30
Nuclear decay is the spontaneous decay of one element into a. an x-ray b. a ray of light c. another element
Answers: 1

Chemistry, 23.06.2019 02:30
Ascientist wants to know how individual lions within a pride interact with each other in their own environment. to do this, the scientist sedates and tags all of the lions within a pride. then, he places several remotely-controlled video cameras near the lions' den and performs an observational field study. he collects continuous video footage over the span of one year, analyzes the video, and then forms conclusions based on his observations.
Answers: 2

Chemistry, 23.06.2019 06:30
The polarity of an oxygen-hydrogen bond is higher than the polarity of a nitrogen-hydrogen bond, allowing amines to be more soluble than alcohols.
Answers: 3
You know the right answer?
Calculate ΔHrxn for the following reaction: Fe2O3(s)+3CO(g)→2Fe(s)+3CO2(g) Use the following reactio...
Questions


Mathematics, 09.01.2020 17:31

Mathematics, 09.01.2020 17:31


Spanish, 09.01.2020 17:31

Mathematics, 09.01.2020 17:31


Mathematics, 09.01.2020 17:31

Biology, 09.01.2020 17:31



English, 09.01.2020 17:31

English, 09.01.2020 17:31



Mathematics, 09.01.2020 17:31




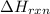 is, -23.9 kJ
is, -23.9 kJ