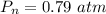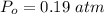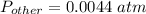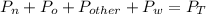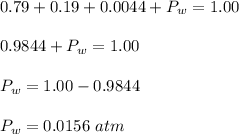Chemistry, 10.03.2020 00:55 ErrorNameTaken505
What is the partial pressure of water vapor in an air sample when the total pressure is 1.00 atm, the partial pressure of nitrogen is 0.79 atm, the partial pressure of oxygen is 0.19 atm, and the partial pressure of all other gases in air is 0.0044 atm?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2


Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1
You know the right answer?
What is the partial pressure of water vapor in an air sample when the total pressure is 1.00 atm, th...
Questions


Mathematics, 31.08.2021 04:30


Mathematics, 31.08.2021 04:30

Mathematics, 31.08.2021 04:30


Mathematics, 31.08.2021 04:30


Mathematics, 31.08.2021 04:30

Mathematics, 31.08.2021 04:30

Mathematics, 31.08.2021 04:30




Mathematics, 31.08.2021 04:30





Mathematics, 31.08.2021 04:30

 .
.