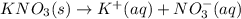Chemistry, 10.03.2020 00:20 blessing5266
The compound potassium nitrate is a strong electrolyte. Write the transformation that occurs when solid potassium nitrate dissolves in water Use the pull-down boxes to specify states such as (aq) or (s).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 17:20
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
The compound potassium nitrate is a strong electrolyte. Write the transformation that occurs when so...
Questions

Mathematics, 10.09.2021 03:50


Mathematics, 10.09.2021 03:50

Biology, 10.09.2021 03:50

Mathematics, 10.09.2021 03:50


Mathematics, 10.09.2021 03:50

Mathematics, 10.09.2021 03:50

Mathematics, 10.09.2021 03:50


Social Studies, 10.09.2021 03:50

Biology, 10.09.2021 03:50


History, 10.09.2021 03:50

Mathematics, 10.09.2021 03:50


Mathematics, 10.09.2021 03:50



Mathematics, 10.09.2021 03:50