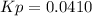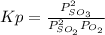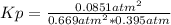Chemistry, 09.03.2020 23:46 TerronRice
A mixture of gaseous sulfur dioxide and oxygen are added to a reaction vessel and heated to 1000 K where they react to form SO3 (g). If the vessel contained 0.669 atm SO2 (g), 0.395 atm O2 (g) and 0.0851 atom SO3 (g) after the system has reached equilibrium, what is the equilibrium constant, Kp, for the reaction

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 23.06.2019 06:40
4786 joules of heat are transferred to a 89.0 gramsample of an unknown material, with an initialtemperature of 23.0°c. what is the specific heat of thematerialif the final temperature is 89.5 °c?
Answers: 1
You know the right answer?
A mixture of gaseous sulfur dioxide and oxygen are added to a reaction vessel and heated to 1000 K w...
Questions