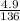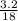Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3
You know the right answer?
8.1grams of xso4.7H2O when heated at 90°c for 15minutes gave 4.9grams when heated further at 120°c....
Questions

Mathematics, 02.12.2021 02:50

Mathematics, 02.12.2021 02:50


History, 02.12.2021 02:50

Biology, 02.12.2021 02:50

Mathematics, 02.12.2021 02:50

English, 02.12.2021 02:50

Mathematics, 02.12.2021 02:50



Social Studies, 02.12.2021 02:50

Computers and Technology, 02.12.2021 02:50

Mathematics, 02.12.2021 02:50

Spanish, 02.12.2021 02:50



Mathematics, 02.12.2021 02:50

Mathematics, 02.12.2021 02:50

Chemistry, 02.12.2021 02:50

Mathematics, 02.12.2021 02:50