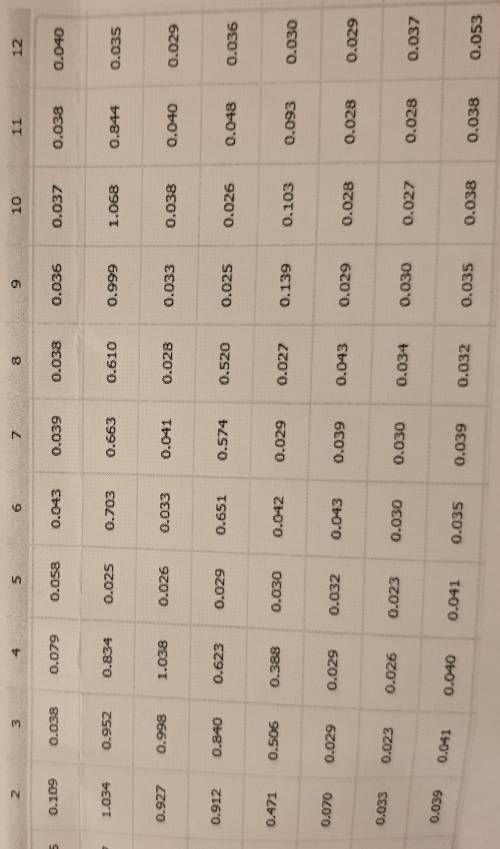
Data Analysis
In Excel, plot the ELISA results using absorbance on the Y axis and th
conce...

Chemistry, 08.03.2020 00:41 TravKeepIt100
Data Analysis
In Excel, plot the ELISA results using absorbance on the Y axis and th
concentrations of the serial dilution samples on the X axis (use a log scale). Th
concentrations of these samples can be calculated from the concentration of th
stock solution. This will be your standard curve. Add a line of best fit to your standard
curve and use the equation of the line to calculate the concentration of ACP
antibodies in your patient and healthy control samples
these are basically my results but how do I do the top question?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:50
2points what is the job of a scientist? a. to answer ethical questions. b. to write laws based on his or her knowledge. c. to ask and answer scientific questions. d. to ignore facts that do not support his or her theory.
Answers: 1

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

You know the right answer?
Questions


Mathematics, 17.11.2020 21:40


Health, 17.11.2020 21:40


Mathematics, 17.11.2020 21:40

English, 17.11.2020 21:40

Mathematics, 17.11.2020 21:40

History, 17.11.2020 21:40


Mathematics, 17.11.2020 21:40


Mathematics, 17.11.2020 21:40

English, 17.11.2020 21:40

Biology, 17.11.2020 21:40

Mathematics, 17.11.2020 21:40

Mathematics, 17.11.2020 21:40

Biology, 17.11.2020 21:40

Chemistry, 17.11.2020 21:40



