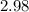Chemistry, 07.03.2020 06:17 missheather0309
A sample of solid NH4NO3 was placed in an evacuated container and then heated so that it decomposed explosively according to the following equation:
NH4NO3(s) N2O(g) + 2H2O(g)
At equilibrium, the total pressure in the container was found to be 2.72 bar at a temperature of 500.°C. Calculate Kp.
a.
1.64
b.
0.822
c.
2.98
d.
80.5
e.
0.745

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3
You know the right answer?
A sample of solid NH4NO3 was placed in an evacuated container and then heated so that it decomposed...
Questions

Computers and Technology, 18.03.2021 23:50



Chemistry, 18.03.2021 23:50


Mathematics, 18.03.2021 23:50

English, 18.03.2021 23:50



Mathematics, 18.03.2021 23:50


Chemistry, 18.03.2021 23:50



History, 18.03.2021 23:50




Social Studies, 18.03.2021 23:50

Mathematics, 18.03.2021 23:50