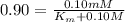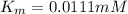Chemistry, 07.03.2020 05:28 jennaaswad088
G In the absence of allosteric effectors, the enzyme phosphofructokinase displays Michaelis–Menten kinetics (see Fig. 7.15). The v0/Vmax ratio is 0.90 when the concentration of the substrate, fructose-6-phosphate, is 0.10 mM. Calculate the KM for phosphofructokinase under these conditions (in units of mM).

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 23.06.2019 02:00
Why does ammonia, nh3, behave as a base when it reacts with an acid? z
Answers: 2
You know the right answer?
G In the absence of allosteric effectors, the enzyme phosphofructokinase displays Michaelis–Menten k...
Questions


Computers and Technology, 19.11.2020 06:00

History, 19.11.2020 06:00

Mathematics, 19.11.2020 06:00

History, 19.11.2020 06:00

Mathematics, 19.11.2020 06:00


English, 19.11.2020 06:00

Mathematics, 19.11.2020 06:00



Mathematics, 19.11.2020 06:00

English, 19.11.2020 06:00


English, 19.11.2020 06:00



Advanced Placement (AP), 19.11.2020 06:00

Arts, 19.11.2020 06:00

![v_o=V_{max}\times \frac{[S]}{(K_m+[S])}=k_{cat}[E_o]\times \frac{[S]}{(K_m+[S])}](/tpl/images/0537/9506/bc195.png)
![V_{max}=k_{cat}[E_o]](/tpl/images/0537/9506/617d3.png)
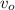 = rate of formation of products
= rate of formation of products ![[K_m]](/tpl/images/0537/9506/99bb2.png) = Michaelis constant
= Michaelis constant 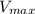 = Maximum rate achieved
= Maximum rate achieved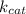 = Catalytic rate of the system
= Catalytic rate of the system ![[E_o]](/tpl/images/0537/9506/e2395.png) = Initial concentration of enzyme
= Initial concentration of enzyme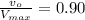
![\frac{v_o}{V_{max}}=\frac{[S]}{(K_m+[S])}](/tpl/images/0537/9506/f52ee.png)