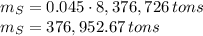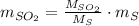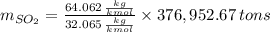Chemistry, 07.03.2020 05:01 qudoniselmore0
In 1986 an electrical power plant in Taylorsville, Georgia, burned 8,376,726 \rm tons of coal, a national record at that time.
Assuming that the coal was 86.0 \% carbon by mass and that combustion was complete, calculate the number of tons of carbon dioxide produced by the plant during the year.
Assuming that the coal was 4.50 \% sulfur by mass and that combustion was complete, calculate the number of tons of sulfur dioxide produced by the plant during the year.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Which of the following best explains why the end of a spoon sticking out of a cup of hot water also gets hot? question 7 options: the heat from the hot water is conducted through the spoon handle the hot water heats the air surrounding the upper part of the spoon. the hot water causes a physical change in the spoon handle. the hot water causes a chemical reaction to take place in the spoon.
Answers: 2


Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3
You know the right answer?
In 1986 an electrical power plant in Taylorsville, Georgia, burned 8,376,726 \rm tons of coal, a nat...
Questions


Mathematics, 04.09.2021 02:00





Mathematics, 04.09.2021 02:00


Mathematics, 04.09.2021 02:00

Mathematics, 04.09.2021 02:00


Mathematics, 04.09.2021 02:00








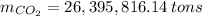 , b)
, b) 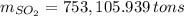
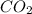 is produced by a mole of
is produced by a mole of 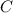 contained in coal. The yearly burnt carbon is:
contained in coal. The yearly burnt carbon is: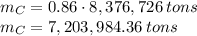
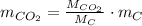
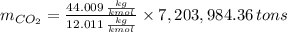
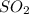 is produced by a mole of
is produced by a mole of 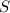 contained in coal.
contained in coal.