

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2
You know the right answer?
Consider the following reaction, equilibrium concentrations, and equilibrium constant at a particula...
Questions

History, 01.03.2021 21:50

Social Studies, 01.03.2021 21:50


Physics, 01.03.2021 21:50

History, 01.03.2021 21:50

Mathematics, 01.03.2021 21:50



History, 01.03.2021 21:50




Computers and Technology, 01.03.2021 21:50


Biology, 01.03.2021 21:50





History, 01.03.2021 21:50

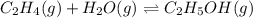
![[C_2H_4]=0.015M](/tpl/images/0537/7510/e5922.png)
![[H_2O]=?](/tpl/images/0537/7510/d3a13.png)
![[C_2H_5OH]=1.69 M](/tpl/images/0537/7510/ae95d.png)
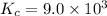
![K_c=\frac{[C_2H_5OH]}{[C_2H_4][H_2O]}](/tpl/images/0537/7510/88e9b.png)
![9.0\times 10^3=\frac{1.69 M}{0.015 M\times [H_2O]}](/tpl/images/0537/7510/9ae4d.png)
![[H_2O]=\frac{1.69 M}{0.015 M\times 9\timers 10^3}=0.0125 M](/tpl/images/0537/7510/e2bbe.png)


