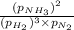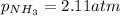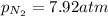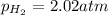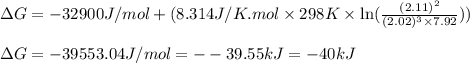Chemistry, 07.03.2020 04:48 Mattisback2285
A chemist fills a reaction vessel with 7.92 atm nitrogen (N2) gas, 2.02 atm hydrogen (H2) gas, and 2.11 atm ammonia (NH3) gas at a temperature of 25.0°C
Under these conditions, calculate the reaction free energy ΔG for the following chemical reaction
N2(8) +3H2 2NH3 (g)
Use the thermodynamic information in the ALEKS Data tab. Round your answer to the nearest kilojoule.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2
You know the right answer?
A chemist fills a reaction vessel with 7.92 atm nitrogen (N2) gas, 2.02 atm hydrogen (H2) gas, and 2...
Questions

History, 14.01.2021 22:00





Mathematics, 14.01.2021 22:00

Social Studies, 14.01.2021 22:00

Mathematics, 14.01.2021 22:00


Mathematics, 14.01.2021 22:00




English, 14.01.2021 22:00


Mathematics, 14.01.2021 22:00

Mathematics, 14.01.2021 22:00

Mathematics, 14.01.2021 22:00

Mathematics, 14.01.2021 22:00

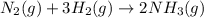
![\Delta G^o_{rxn}=[(2\times \Delta G^o_f_{(NH_3(g))})]-[(1\times \Delta G^o_f_{(N_2(g))})+(3\times \Delta G^o_f_{(H_2(g))})]](/tpl/images/0537/7676/6e73c.png)
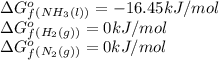
![\Delta G^o_{rxn}=[(2\times (-16.45))]-[(1\times (0))+(3\times (0))]\\\\\Delta G^o_{rxn}=-32.9kJ/mol](/tpl/images/0537/7676/8bdb6.png)
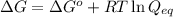
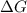 = free energy of the reaction
= free energy of the reaction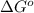 = standard Gibbs free energy = -32.9 kJ/mol = -32900 J/mol (Conversion factor: 1 kJ = 1000 J)
= standard Gibbs free energy = -32.9 kJ/mol = -32900 J/mol (Conversion factor: 1 kJ = 1000 J)![25^oC=[273+25]K=298K](/tpl/images/0537/7676/0e82f.png)
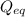 = Ratio of concentration of products and reactants at any time =
= Ratio of concentration of products and reactants at any time =