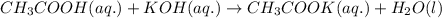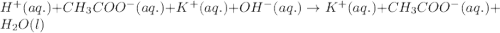Chemistry, 07.03.2020 04:57 BlehBlehBlehBleh
Equal volumes of 0.25 M acetic acid and 0.25 M potassium hydroxide are combined. Write the net ionic equation for the reaction and identify the aqueous species that have the highest concentrations at equilibrium. Justify your answer.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Aside from human impact, which of the following causes less water vapor production over a small area? (2 pderivartin
Answers: 1

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1

Chemistry, 23.06.2019 12:50
How many energy levels contain electrons in an atom of zirconium (zr)?
Answers: 1
You know the right answer?
Equal volumes of 0.25 M acetic acid and 0.25 M potassium hydroxide are combined. Write the net ionic...
Questions


Chemistry, 30.07.2019 07:30


Mathematics, 30.07.2019 07:30


Physics, 30.07.2019 07:30

Mathematics, 30.07.2019 07:30

Mathematics, 30.07.2019 07:30



Chemistry, 30.07.2019 07:30


Mathematics, 30.07.2019 07:30

Mathematics, 30.07.2019 07:30

Mathematics, 30.07.2019 07:30



Mathematics, 30.07.2019 07:30


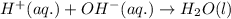 and all the ions have equal concentrations at equilibrium
and all the ions have equal concentrations at equilibrium