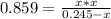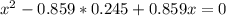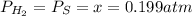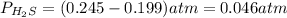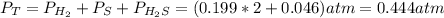Answers: 3


Another question on Chemistry

Chemistry, 20.06.2019 18:04
Two liquids are shaken together in a test tube to produce a mixture that quickly separates into two layers. which of the following best describes the behavior of the above pair of substances? soluble insoluble miscible immiscible
Answers: 1

Chemistry, 21.06.2019 13:00
One significant difference between an ionic bond, where electrons are taken from one atom and added to another atom, and a covalent or metallic bond, where electrons are shared is
Answers: 2

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1
You know the right answer?
At a certain temperature, the K p for the decomposition of H 2 S is 0.859 . H 2 S ( g ) − ⇀ ↽ − H 2...
Questions

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Computers and Technology, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Social Studies, 17.09.2020 14:01

Arts, 17.09.2020 14:01

Chemistry, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Physics, 17.09.2020 14:01

Social Studies, 17.09.2020 14:01

Physics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

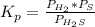 (1)
(1)