
Chemistry, 07.03.2020 03:58 isaiahcannon5709
A certain substance has a heat of vaporization of 70.83 kJ / mol. 70.83 kJ/mol. At what Kelvin temperature will the vapor pressure be 5.00 5.00 times higher than it was at 331 K?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2
You know the right answer?
A certain substance has a heat of vaporization of 70.83 kJ / mol. 70.83 kJ/mol. At what Kelvin tempe...
Questions





Mathematics, 30.01.2020 18:55

Mathematics, 30.01.2020 18:55


Chemistry, 30.01.2020 18:55

Computers and Technology, 30.01.2020 18:55



History, 30.01.2020 18:55

Mathematics, 30.01.2020 18:55

Mathematics, 30.01.2020 18:55

History, 30.01.2020 18:55

History, 30.01.2020 18:55

Health, 30.01.2020 18:55



Biology, 30.01.2020 18:55

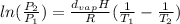
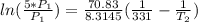 =
= 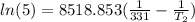 or T₂ =
or T₂ = 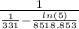 = 353.0797 K
= 353.0797 K

