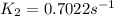Chemistry, 07.03.2020 04:09 beelcypher
The rate constant of a first-order reaction is 3.46 × 10−2 s−1 at 298 K. What is the rate constant at 350 K if the activation energy for the reaction is 50.2 kJ/mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 23.06.2019 01:50
Drag the tiles to the correct locations. each tile can be used more than once, but not all tiles will be used. one or more locations will remain empty. nitrosyl fluoride has the chemical formula nof nitrogen has five valence electrons, oxygen has six, and fluorine has seven. complete the lewis structure for this covalent compound. f n = = = . : : 0 : reset next um. all rights reserved us 2
Answers: 2
You know the right answer?
The rate constant of a first-order reaction is 3.46 × 10−2 s−1 at 298 K. What is the rate constant a...
Questions

History, 09.04.2020 02:20

Mathematics, 09.04.2020 02:20

Mathematics, 09.04.2020 02:20


Physics, 09.04.2020 02:20



Mathematics, 09.04.2020 02:20

Mathematics, 09.04.2020 02:20

Biology, 09.04.2020 02:20


English, 09.04.2020 02:20


Business, 09.04.2020 02:21

Social Studies, 09.04.2020 02:21

Social Studies, 09.04.2020 02:21




English, 09.04.2020 02:21

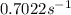 is the rate constant at 350 K if the activation energy for the reaction is 50.2 kJ/mol.
is the rate constant at 350 K if the activation energy for the reaction is 50.2 kJ/mol.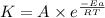
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0537/5727/6d953.png)
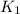 = rate constant at 298 K=
= rate constant at 298 K= 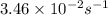
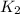 = rate constant at 350 K =?
= rate constant at 350 K =?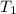 = initial temperature = 298 K
= initial temperature = 298 K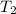 = final temperature = 350 K
= final temperature = 350 K![\log (\frac{K_2}{3.46\times 10^{-2} s^{-1}})=\frac{50200 J/mol}{2.303\times 8.314J/mole.K}[\frac{1}{350 K}-\frac{1}{298 K}]](/tpl/images/0537/5727/978f6.png)