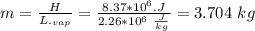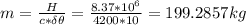Chemistry, 07.03.2020 03:56 damiangibson2
Assume that a daily diet of 2000 calories (i. e. 8.37 x 106 J) is converted completely to body heat.
a) How many g of water (as sweat) would need to evaporate to cool that person off?
b) If instead of evaporating water, the heat was used to raise the temperature of some water from 25.0 °C to 35.0 °C, how much water could be heated?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
You know the right answer?
Assume that a daily diet of 2000 calories (i. e. 8.37 x 106 J) is converted completely to body heat....
Questions


Computers and Technology, 16.01.2021 23:40



Mathematics, 16.01.2021 23:40

History, 16.01.2021 23:40

Mathematics, 16.01.2021 23:40

Chemistry, 16.01.2021 23:40

English, 16.01.2021 23:40


History, 16.01.2021 23:40

Mathematics, 16.01.2021 23:40


Mathematics, 16.01.2021 23:40


Mathematics, 16.01.2021 23:40