
Chemistry, 07.03.2020 04:14 mckleinrivero
The rate constant for a certain reaction is kkk = 3.50×10−3 s−1s−1 . If the initial reactant concentration was 0.450 MM, what will the concentration be after 19.0 minutes?
A zero-order reaction has a constant rate of 3.50×10−4 M/sM/s. If after 65.0 seconds the concentration has dropped to 3.50×10−2 MM, what was the initial concentration?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2


Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1
You know the right answer?
The rate constant for a certain reaction is kkk = 3.50×10−3 s−1s−1 . If the initial reactant concent...
Questions



Mathematics, 30.10.2020 01:30

English, 30.10.2020 01:30

Mathematics, 30.10.2020 01:30



Mathematics, 30.10.2020 01:30

Mathematics, 30.10.2020 01:30






Mathematics, 30.10.2020 01:30

Mathematics, 30.10.2020 01:30

Mathematics, 30.10.2020 01:30

Mathematics, 30.10.2020 01:30


![Rate = k[A][B]](/tpl/images/0537/5969/ab111.png) . As the data are given in the question, the rate constant is
. As the data are given in the question, the rate constant is 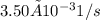 , the initial concentration
, the initial concentration![[A]_o = 0.450 M](/tpl/images/0537/5969/1d14c.png) and time is 19 minutes that is the 1140s.
and time is 19 minutes that is the 1140s.
![ln[A] = ln[A]_o - kt\\ln[A] = ln (0.450) - (3.50*10^-^3)1140\\ln[A] = -0.799 - 3.99\\ln[A] = -4.789\\[A] = 0.0083M](/tpl/images/0537/5969/91bfd.png)
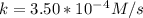 Time = 65 s
Final Concentration [A] =
Time = 65 s
Final Concentration [A] = 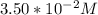
![[A] = [A]_o - kt[A]_o = [A] + kt\\[A]o = 3.50*10^{-2} + (3.50*10^{-4}) * 65\\[A]_o = 3.50*10^{-2} + 0.02275\\[A]_o = 0.05775 M](/tpl/images/0537/5969/3e5cb.png)


