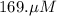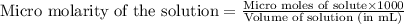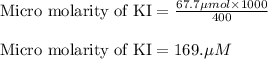Chemistry, 07.03.2020 03:40 ayoismeisalex
A chemist prepares a solution of potassium iodide (KI) by measuring out 67.7. mu mol of potassium iodide into a 400. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mmo1L of the chemist's potassium iodide solution. Round your answer to 3 significant digits.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 23.06.2019 03:00
A0.100-kilogram apple hangs in a tree 1.50 meter above the ground. ignore frictional effects, the total mechanical energy of the apples is
Answers: 1
You know the right answer?
A chemist prepares a solution of potassium iodide (KI) by measuring out 67.7. mu mol of potassium io...
Questions



Biology, 13.05.2021 05:00

Chemistry, 13.05.2021 05:00






History, 13.05.2021 05:00







Mathematics, 13.05.2021 05:00

Mathematics, 13.05.2021 05:00

Mathematics, 13.05.2021 05:00

Mathematics, 13.05.2021 05:00