
Chemistry, 07.03.2020 03:49 queenkimm26
Hydrogen iodide decomposes slowly to H2 and I2 at 600 K. The reaction is second order in HI, and the rate constant is 9.7×10−6M−1s−1. If the initial concentration of HI is 0.110 M. What is its molarity after a reaction time of 5.00 days? Express your answer in moles per liter to two significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3

You know the right answer?
Hydrogen iodide decomposes slowly to H2 and I2 at 600 K. The reaction is second order in HI, and the...
Questions


Health, 18.03.2021 02:50


Mathematics, 18.03.2021 02:50



Mathematics, 18.03.2021 02:50


World Languages, 18.03.2021 02:50




Mathematics, 18.03.2021 02:50

Arts, 18.03.2021 02:50



Business, 18.03.2021 02:50

English, 18.03.2021 02:50

English, 18.03.2021 02:50

Physics, 18.03.2021 02:50

![k=\frac{1}{t}\left (\frac{1}{[A]}-\frac{1}{[A]_o}\right)](/tpl/images/0537/4912/5ea71.png)
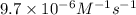
![[A]_o](/tpl/images/0537/4912/9caf5.png) = Initial concentration = 0.110 M
= Initial concentration = 0.110 M![9.7\times 10^{-6}=\frac{1}{5.00}\left (\frac{1}{[A]}-\frac{1}{(0.110)}\right)](/tpl/images/0537/4912/07474.png)
![[A]=0.109M](/tpl/images/0537/4912/e5264.png)


