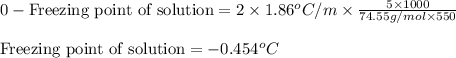Chemistry, 07.03.2020 03:18 dontcareanyonemo
Calculate the freezing point of a solution containing 5.0 grams of KCl and 550.0 grams of water. The molal-freezing-point-depression constant () for water is 1.86

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 13:00
Lab reagent, hypothesis test.a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl.these six measurements are assumed to be an srs of all possible measurements from solution.they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution.carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1
You know the right answer?
Calculate the freezing point of a solution containing 5.0 grams of KCl and 550.0 grams of water. The...
Questions

Mathematics, 18.09.2020 23:01

Social Studies, 18.09.2020 23:01

Social Studies, 18.09.2020 23:01

Mathematics, 18.09.2020 23:01

Mathematics, 18.09.2020 23:01

Mathematics, 18.09.2020 23:01

Mathematics, 18.09.2020 23:01

Mathematics, 18.09.2020 23:01

Mathematics, 18.09.2020 23:01

Mathematics, 18.09.2020 23:01

Mathematics, 18.09.2020 23:01

English, 18.09.2020 23:01

Mathematics, 18.09.2020 23:01

Social Studies, 18.09.2020 23:01

Mathematics, 18.09.2020 23:01

Mathematics, 18.09.2020 23:01

Mathematics, 18.09.2020 23:01

Health, 19.09.2020 01:01



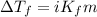
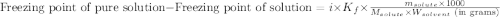
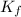 = molal freezing point elevation constant = 1.86°C/m
= molal freezing point elevation constant = 1.86°C/m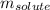 = Given mass of solute (KCl) = 5.0 g
= Given mass of solute (KCl) = 5.0 g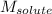 = Molar mass of solute (KCl) = 74.55 g/mol
= Molar mass of solute (KCl) = 74.55 g/mol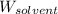 = Mass of solvent (water) = 550.0 g
= Mass of solvent (water) = 550.0 g