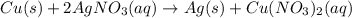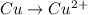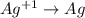Chemistry, 07.03.2020 03:06 chutchins0406
Dentify the oxidized substance, the reduced substance, the oxidizing agent, and the reducing agent in the redox reaction. Cu ( s ) + 2 AgNO 3 ( aq ) ⟶ 2 Ag ( s ) + Cu ( NO 3 ) 2 ( aq )

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:20
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3
You know the right answer?
Dentify the oxidized substance, the reduced substance, the oxidizing agent, and the reducing agent i...
Questions



Geography, 05.10.2019 16:00

Chemistry, 05.10.2019 16:00

Social Studies, 05.10.2019 16:00


Mathematics, 05.10.2019 16:00

English, 05.10.2019 16:00

Mathematics, 05.10.2019 16:00

English, 05.10.2019 16:00

History, 05.10.2019 16:00

Geography, 05.10.2019 16:00

Computers and Technology, 05.10.2019 16:00


English, 05.10.2019 16:00

Mathematics, 05.10.2019 16:00

History, 05.10.2019 16:00

History, 05.10.2019 16:00


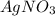 act as oxidizing agent and Cu act as an reducing agent.
act as oxidizing agent and Cu act as an reducing agent.