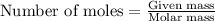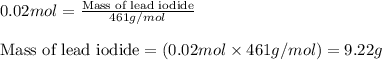Chemistry, 07.03.2020 02:57 graymonky12
Write the balanced equation for the reaction of aqueous Pb ( ClO 3 ) 2 with aqueous NaI . Include phases. chemical equation: What mass of precipitate will form if 1.50 L of highly concentrated Pb ( ClO 3 ) 2 is mixed with 0.200 L 0.200 M NaI ? Assume the reaction goes to completion.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Arock can be broken down into different kinds of substances by physical processes. no chemical reactions are needed to separate different parts of a rock into pure substances. this is because a rock is a(n)
Answers: 1

Chemistry, 21.06.2019 17:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5 m hcl? show all of the work needed to solve this problem. mg (s) + 2hcl (aq) → mgcl2 (aq) + h2 (g)
Answers: 3

Chemistry, 21.06.2019 18:40
What kind of ion is contained in salts that produce an acidic solution? a positive ion that attracts a proton from water a positive ion that releases a proton to water a negative ion that attracts a proton from water a negative ion that releases a proton to water
Answers: 1

Chemistry, 21.06.2019 18:50
Which term refers to a property that depends only on the amount of a substance? ©@
Answers: 2
You know the right answer?
Write the balanced equation for the reaction of aqueous Pb ( ClO 3 ) 2 with aqueous NaI . Include ph...
Questions

Biology, 05.03.2021 03:30

Mathematics, 05.03.2021 03:30


Mathematics, 05.03.2021 03:30

Mathematics, 05.03.2021 03:30

Mathematics, 05.03.2021 03:30

Computers and Technology, 05.03.2021 03:30

Mathematics, 05.03.2021 03:30

Mathematics, 05.03.2021 03:30

Mathematics, 05.03.2021 03:30

Mathematics, 05.03.2021 03:30

Mathematics, 05.03.2021 03:30

Mathematics, 05.03.2021 03:30

Mathematics, 05.03.2021 03:30

Chemistry, 05.03.2021 03:30

Mathematics, 05.03.2021 03:30

Social Studies, 05.03.2021 03:30



Mathematics, 05.03.2021 03:30

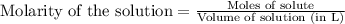
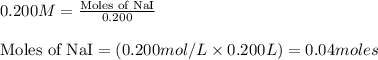
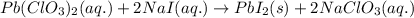
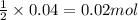 of lead iodide
of lead iodide