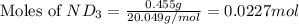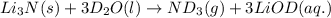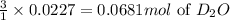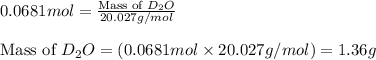Lithium nitride reacts with water to produce ammonia and lithium hydroxide according to the equation Li 3 N ( s ) + 3 H 2 O ( l ) ⟶ NH 3 ( g ) + 3 LiOH ( aq ) Heavy water is water with the isotope deuterium in place of ordinary hydrogen, and its formula is D 2 O . The same reaction can be used to produce heavy ammonia, ND 3 ( g ) , according to the equation Li 3 N ( s ) + 3 D 2 O ( l ) ⟶ ND 3 ( g ) + 3 LiOD ( aq ) Calculate how many grams of heavy water are required to produce 455.0 mg ND 3 ( g ) . The mass of deuterium, D , is 2.014 g/mol.

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 00:30
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2

Chemistry, 23.06.2019 03:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1


Chemistry, 23.06.2019 03:30
How many grams of sodium chloride are in 250ml of a 2.5m naci solution
Answers: 1
You know the right answer?
Lithium nitride reacts with water to produce ammonia and lithium hydroxide according to the equation...
Questions

English, 28.12.2019 06:31

History, 28.12.2019 06:31

Mathematics, 28.12.2019 06:31

Mathematics, 28.12.2019 06:31

Chemistry, 28.12.2019 06:31


Mathematics, 28.12.2019 06:31


Spanish, 28.12.2019 06:31


Mathematics, 28.12.2019 06:31






English, 28.12.2019 06:31


History, 28.12.2019 06:31

History, 28.12.2019 06:31

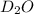 needed is 1.36 grams
needed is 1.36 grams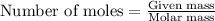 .....(1)
.....(1) :
: