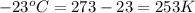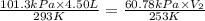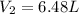Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2
You know the right answer?
A helium balloon with an internal pressure of 1.0 atm and a volume of 4.50 L at 20.0⁰C is released....
Questions


Mathematics, 23.08.2019 14:10




Mathematics, 23.08.2019 14:10

English, 23.08.2019 14:10

English, 23.08.2019 14:10


English, 23.08.2019 14:10


English, 23.08.2019 14:10


Social Studies, 23.08.2019 14:10

Mathematics, 23.08.2019 14:10

Health, 23.08.2019 14:10



History, 23.08.2019 14:10

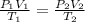
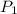 = initial pressure of gas = 1 atm = 101.3 kPa
= initial pressure of gas = 1 atm = 101.3 kPa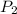 = final pressure of gas = 60.78 kPa
= final pressure of gas = 60.78 kPa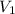 = initial volume of gas = 4.50 L
= initial volume of gas = 4.50 L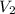 = final volume of gas = ?
= final volume of gas = ?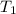 = initial temperature of gas =
= initial temperature of gas = 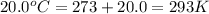
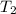 = final temperature of gas =
= final temperature of gas =