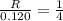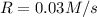Chemistry, 07.03.2020 02:49 minecraft37385
At a certain concentration of H2 and NH3, the initial rate of reaction is 0.120 M / s. What would the initial rate of the reaction be if the concentration of H2 were halved

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

You know the right answer?
At a certain concentration of H2 and NH3, the initial rate of reaction is 0.120 M / s. What would th...
Questions


Biology, 31.07.2019 02:40

Biology, 31.07.2019 02:40

Biology, 31.07.2019 02:40

History, 31.07.2019 02:40

Business, 31.07.2019 02:40

Business, 31.07.2019 02:40

History, 31.07.2019 02:40



Mathematics, 31.07.2019 02:40








Biology, 31.07.2019 02:40

Biology, 31.07.2019 02:40

![rate=k[H_2]^2[NH_3]](/tpl/images/0537/2256/8fdd7.png)
![H_2 and [tex]I_2, the initial rate of reaction is 0.120 M/s. What would the initial rate of the reaction be if the concentration of [tex]H_2 were halved.Answer : The initial rate of the reaction will be, 0.03 M/sExplanation :Rate law expression for the reaction:[tex]rate=k[H_2]^2[NH_3]](/tpl/images/0537/2256/23b7f.png)
![0.120=k[H_2]^2[NH_3]](/tpl/images/0537/2256/be6ca.png) ....(1)
....(1)![R=k(\frac{[H_2]}{2})^2[NH_3]](/tpl/images/0537/2256/dca92.png) ....(2)
....(2)![\frac{R}{0.120}=\frac{k(\frac{[H_2]}{2})^2[NH_3]}{k[H_2]^2[NH_3]}](/tpl/images/0537/2256/6c355.png)