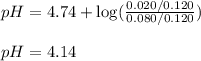Chemistry, 07.03.2020 01:18 kingbudd129
Calculate the pH of adding 20 mL of 1 M NaOH solution to 100 mL of a 1 M acetic acid (CH3COOH) solution and 880 mL of distilled water. Assume that the Ka of acetic acid is 1.8 * 10-5

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1

Chemistry, 23.06.2019 16:30
Ashell can hold a maximum of 32 electrons, what is the value of n?
Answers: 3
You know the right answer?
Calculate the pH of adding 20 mL of 1 M NaOH solution to 100 mL of a 1 M acetic acid (CH3COOH) solut...
Questions


Mathematics, 29.11.2020 06:50

English, 29.11.2020 06:50


Business, 29.11.2020 06:50


History, 29.11.2020 06:50


History, 29.11.2020 06:50

Mathematics, 29.11.2020 06:50





English, 29.11.2020 06:50



Mathematics, 29.11.2020 07:00

Mathematics, 29.11.2020 07:00

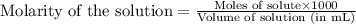
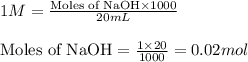
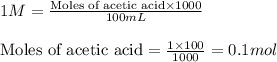
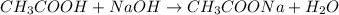
![pH=pK_a+\log(\frac{[salt]}{[acid]})](/tpl/images/0536/9084/e4eea.png)
![pH=pK_a+\log(\frac{[CH_3COONa]}{[CH_3COOH]})](/tpl/images/0536/9084/05ea7.png)
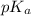 = negative logarithm of acid dissociation constant of acetic acid = 4.74
= negative logarithm of acid dissociation constant of acetic acid = 4.74![[CH_3COONa]=\frac{0.020}{0.120}](/tpl/images/0536/9084/69ac9.png)
![[CH_3COOH]=\frac{0.080}{0.120}](/tpl/images/0536/9084/afc8b.png)