

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 23.06.2019 00:30
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1

Chemistry, 23.06.2019 01:30
Ascientist is measuring the pressure that is exerted by each of the following gases in the atmosphere: carbon dioxide, oxygen, and nitrogen. which term most likely describes what she is measuring?
Answers: 1
You know the right answer?
The rate constant of a certain reaction is known to obey the Arrhenius equation, and to have an acti...
Questions

History, 07.10.2021 16:40


Mathematics, 07.10.2021 16:40

Arts, 07.10.2021 16:40

Computers and Technology, 07.10.2021 16:40

Social Studies, 07.10.2021 16:40

Social Studies, 07.10.2021 16:40

Biology, 07.10.2021 16:40






Mathematics, 07.10.2021 16:40


Mathematics, 07.10.2021 16:40

Geography, 07.10.2021 16:40


Social Studies, 07.10.2021 16:40

Chemistry, 07.10.2021 16:50

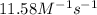
![\ln(\frac{K_{56.0^oC}}{K_{-8.0^oC}})=\frac{E_a}{R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0536/9043/5e447.png)
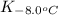 = equilibrium constant at -8.0°C =
= equilibrium constant at -8.0°C = 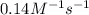
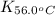 = equilibrium constant at 56°C = ?
= equilibrium constant at 56°C = ?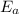 = Activation energy = 50.0 kJ/mol = 50000 J/mol (Conversion factor: 1 kJ = 1000 J)
= Activation energy = 50.0 kJ/mol = 50000 J/mol (Conversion factor: 1 kJ = 1000 J)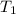 = initial temperature =
= initial temperature = ![-8.0^oC=[273-8]K=265K](/tpl/images/0536/9043/f4197.png)
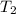 = final temperature =
= final temperature = ![56.0^oC=[273+56]K=329K](/tpl/images/0536/9043/23b22.png)
![\ln(\frac{K_{56.0^oC}}{0.14})=\frac{50000J}{8.314J/mol.K}[\frac{1}{265}-\frac{1}{329}]\\\\K_{56.0^oC}=11.58M^{-1}s^{-1}](/tpl/images/0536/9043/35497.png)



