

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1

You know the right answer?
For the imaginary element abdicinium (Ab), two isotopes exist. Isotope one has a mass of 40.005 amu...
Questions




Mathematics, 13.01.2021 01:00

Mathematics, 13.01.2021 01:00


Mathematics, 13.01.2021 01:00

English, 13.01.2021 01:00







Mathematics, 13.01.2021 01:00



Chemistry, 13.01.2021 01:00

Mathematics, 13.01.2021 01:00

Mathematics, 13.01.2021 01:00

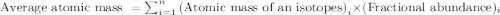 .....(1)
.....(1)![\text{Average atomic mass of abdicinium}=[(40.005\times 0.1400)+(41.008\times 0.8600)]\\\\\text{Average atomic mass of abdicinium}=40.8676amu](/tpl/images/0536/7110/ae4a8.png)


