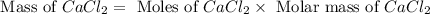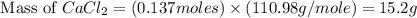Chemistry, 07.03.2020 00:15 IBillRandomz2958
When calcium carbonate is added to hydrochloric acid, calcium chloride, carbon dioxide, and water are produced. CaCO3 ( s ) + 2 HCl ( aq ) ⟶ CaCl2 ( aq ) + H 2 O ( l ) + CO 2 ( g ) How many grams of calcium chloride will be produced when 30.0 g of calcium carbonate is combined with 10.0 g of hydrochloric acid

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1
You know the right answer?
When calcium carbonate is added to hydrochloric acid, calcium chloride, carbon dioxide, and water ar...
Questions




Mathematics, 19.08.2019 12:50



Social Studies, 19.08.2019 12:50

Social Studies, 19.08.2019 12:50





Mathematics, 19.08.2019 12:50

Mathematics, 19.08.2019 12:50

Social Studies, 19.08.2019 12:50


English, 19.08.2019 12:50

Mathematics, 19.08.2019 12:50

Mathematics, 19.08.2019 12:50

Mathematics, 19.08.2019 12:50

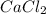 produced is, 15.2 grams.
produced is, 15.2 grams. = 30.0 g
= 30.0 g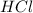 = 10.0 g
= 10.0 g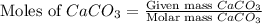
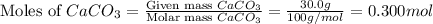
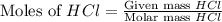
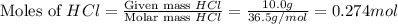
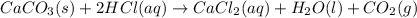
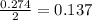 moles of
moles of