
Chemistry, 06.03.2020 23:26 lilquongohard
You carefully weigh out 16.00 g of CaCO3 powder and add it to 64.80 g of HCl solution. You notice bubbles as a reaction takes place. You then weigh the resulting solution and find that it has a mass of 74.24 g . The relevant equation is CaCO_3(s) + 2HCl(aq) rightarrow H_2O(l) + CO_2(g) + CaCl_2(aq) Assuming no other reactions take place, what mass of CO_2 was produced in this reaction?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 23.06.2019 00:20
4. propanol and isopropanol are isomers. this means that they have a) the same molecular formula but different chemical properties. b) different molecular formulas but the same chemical properties. c) the same molecular formula and the same chemical properties. d) the same molecular formula but represent different states of the compound
Answers: 3

Chemistry, 23.06.2019 03:00
What happens in the particles of a gas when the gas is compressed
Answers: 1

Chemistry, 23.06.2019 06:00
Which factor is likely to impact the possible number of compounds? presence of unlimited number of elements in the periodic table the inability of atoms to align perfectly with other atoms the ability of all elements to react with every other element all elements being equally reactive
Answers: 2
You know the right answer?
You carefully weigh out 16.00 g of CaCO3 powder and add it to 64.80 g of HCl solution. You notice bu...
Questions


History, 01.10.2019 21:00



History, 01.10.2019 21:00


Mathematics, 01.10.2019 21:00


Biology, 01.10.2019 21:00

History, 01.10.2019 21:00

Business, 01.10.2019 21:00

English, 01.10.2019 21:00


Mathematics, 01.10.2019 21:00

Mathematics, 01.10.2019 21:00


Mathematics, 01.10.2019 21:00


Social Studies, 01.10.2019 21:00

Biology, 01.10.2019 21:00

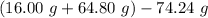
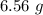
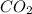 produced in this reaction was 6.56 grams
produced in this reaction was 6.56 grams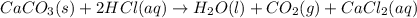
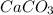 + mass of
+ mass of 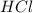 = 16.00 + 64.80 = 80.80 g
= 16.00 + 64.80 = 80.80 g

