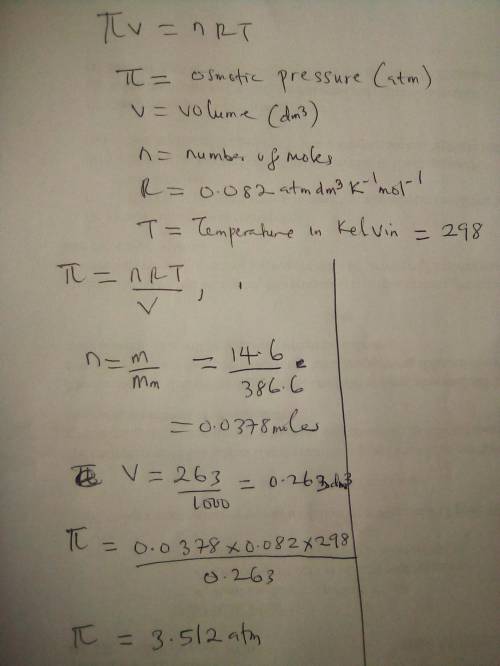Chemistry, 06.03.2020 19:06 teamzomaxx6584
The nonvolatile, nonelectrolyte cholesterol, C27H46O (386.60 g/mol), is soluble in benzene C6H6. Calculate the osmotic pressure generated when 14.6 grams of cholesterol are dissolved in 263 ml of a benzene solution at 298 K. The molarity of the solution is M. The osmotic pressure of the solution is atmospheres.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1
You know the right answer?
The nonvolatile, nonelectrolyte cholesterol, C27H46O (386.60 g/mol), is soluble in benzene C6H6. Cal...
Questions

Physics, 19.08.2019 03:20

Mathematics, 19.08.2019 03:20

Mathematics, 19.08.2019 03:20


Social Studies, 19.08.2019 03:20

Mathematics, 19.08.2019 03:20

History, 19.08.2019 03:20

English, 19.08.2019 03:20



Mathematics, 19.08.2019 03:20




Mathematics, 19.08.2019 03:20

English, 19.08.2019 03:20




Physics, 19.08.2019 03:20