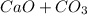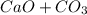Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 23:30
What are the similarities between compounds and mixtures?
Answers: 3
You know the right answer?
Problem Page Question Solid calcium carbonate decomposes into solid calcium oxide and carbon dioxide...
Questions

Mathematics, 24.11.2019 20:31

Biology, 24.11.2019 20:31


Social Studies, 24.11.2019 20:31

English, 24.11.2019 20:31




Biology, 24.11.2019 20:31

History, 24.11.2019 20:31



Mathematics, 24.11.2019 20:31



Mathematics, 24.11.2019 20:31

Mathematics, 24.11.2019 20:31



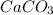 →
→