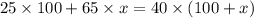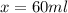Chemistry, 06.03.2020 09:16 raulhill98
You need a 40% alcohol solution. On hand, you have a 100 mL of a 25% alcohol mixture. You also have 65% alcohol mixture. How much of the 65% mixture will you need to add to obtain the desired solution

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

You know the right answer?
You need a 40% alcohol solution. On hand, you have a 100 mL of a 25% alcohol mixture. You also have...
Questions

Biology, 25.11.2020 17:40




Arts, 25.11.2020 17:40

Mathematics, 25.11.2020 17:40



Geography, 25.11.2020 17:40

Advanced Placement (AP), 25.11.2020 17:40


Mathematics, 25.11.2020 17:40



Mathematics, 25.11.2020 17:40

Mathematics, 25.11.2020 17:40

Computers and Technology, 25.11.2020 17:50

Mathematics, 25.11.2020 17:50

Social Studies, 25.11.2020 17:50

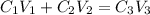
 = volume of given alcohol solution = 100 ml
= volume of given alcohol solution = 100 ml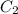 = concentration of another alcohol solution=65 %
= concentration of another alcohol solution=65 %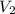 = volume of another acid solution= x ml
= volume of another acid solution= x ml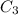 = concentration of resulting alcohol solution = 40 %
= concentration of resulting alcohol solution = 40 %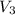 = volume of resulting acid solution = (100+x)
= volume of resulting acid solution = (100+x)