Chemistry, 06.03.2020 01:48 GhostElite6383
Ammonia will decompose into nitrogen and hydrogen at high temperature. An industrial chemist studying this reaction fills a 7.50 l tank with 29.0 mol of ammonia gas at 35.0 °C. She then raises the temperature, and when the mixture has come to equilibrium measures the amount of nitrogen gas to be 13.0 mol.
Calculate the concentration equilibrium constant for the decomposition of ammonia at the final temperature of the mixture. Round your answer to significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2


Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1
You know the right answer?
Ammonia will decompose into nitrogen and hydrogen at high temperature. An industrial chemist studyin...
Questions

Biology, 30.09.2019 08:30

Mathematics, 30.09.2019 08:30

English, 30.09.2019 08:30


English, 30.09.2019 08:30

Geography, 30.09.2019 08:30




Mathematics, 30.09.2019 08:30


Mathematics, 30.09.2019 08:30




Mathematics, 30.09.2019 08:30


Computers and Technology, 30.09.2019 08:30

History, 30.09.2019 08:30

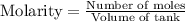
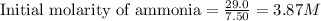
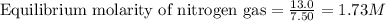
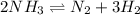
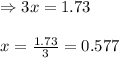
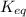 for above equation follows:
for above equation follows:![K_{eq}=\frac{[N_2][H_2]^3}{[NH_3]^2}](/tpl/images/0535/0025/804f3.png)