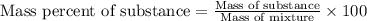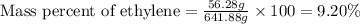Ethylene () is the starting point for a wide array of industrial chemical syntheses. For example, worldwide about of polyethylene are made from ethylene each year, for use in everything from household plumbing to artificial joints. Natural sources of ethylene are entirely inadequate to meet world demand, so ethane () from natural gas is "cracked" in refineries at high temperature in a kinetically complex reaction that produces ethylene gas and hydrogen gas. Suppose an engineer studying ethane cracking fills a reaction tank with of ethane gas and raises the temperature to . He believes at this temperature. Calculate the percent by mass of ethylene the engineer expects to find in the equilibrium gas mixture. Round your answer to significant digits. Note for advanced students: the engineer may be mistaken about the correct value of , and the mass percent of ethylene you calculate may not be what he actually observes.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2


Chemistry, 23.06.2019 00:20
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1

Chemistry, 23.06.2019 06:30
What type of chemical reaction occurs between silver nitrate (agno3) and copper (cu)? the equation i was given is 2agno3 + cu —> 2ag+ cu(no3)2.
Answers: 1
You know the right answer?
Ethylene () is the starting point for a wide array of industrial chemical syntheses. For example, wo...
Questions

Mathematics, 21.07.2019 09:30







Biology, 21.07.2019 09:30


Social Studies, 21.07.2019 09:30

Biology, 21.07.2019 09:30

Social Studies, 21.07.2019 09:30


Biology, 21.07.2019 09:30


Social Studies, 21.07.2019 09:30

Mathematics, 21.07.2019 09:30


Mathematics, 21.07.2019 09:30

History, 21.07.2019 09:30

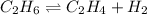
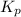 for above equation follows:
for above equation follows: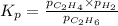
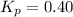
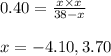
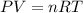 ..........(1)
..........(1)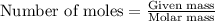 .....(2)
.....(2)![P=34.3atm\\V=30.0L\\R=0.0821\text{ L atm }mol^{-1}K^{-1}\\T=400^oC=[400+273]=673K](/tpl/images/0534/8160/3194c.png)

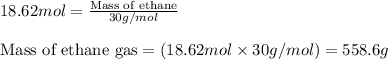
![P=3.70atm\\V=30.0L\\R=0.0821\text{ L atm }mol^{-1}K^{-1}\\T=400^oC=[400+273]=673K](/tpl/images/0534/8160/4b69b.png)