Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
A solution is prepared by dissolving 27.0 g of urea [(NH2)2CO], in 150.0 g of water. Calculate the b...
Questions

Mathematics, 12.02.2021 02:20

Mathematics, 12.02.2021 02:20


Social Studies, 12.02.2021 02:20




Mathematics, 12.02.2021 02:20

Mathematics, 12.02.2021 02:20

Mathematics, 12.02.2021 02:20





Computers and Technology, 12.02.2021 02:20


English, 12.02.2021 02:20



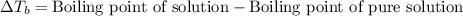
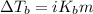
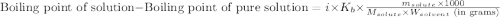
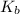 = molal boiling point elevation constant = 0.52°C/m.g
= molal boiling point elevation constant = 0.52°C/m.g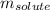 = Given mass of solute (urea) = 27.0 g
= Given mass of solute (urea) = 27.0 g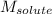 = Molar mass of solute (urea) = 60 g/mol
= Molar mass of solute (urea) = 60 g/mol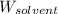 = Mass of solvent (water) = 150.0 g
= Mass of solvent (water) = 150.0 g