
Chemistry, 05.03.2020 03:40 joThompson
When this system is at equilibrium at a certain temperature PCl5(g) ⇋ PCl3(g) + Cl2(g), the concentrations are found to be [PCl5] = 0.40 M, [PCl3] = [Cl2] = 0.20. If the volume of the container is suddenly halved at the same temperature, what will be the new equilibrium concentration of PCl5?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3

You know the right answer?
When this system is at equilibrium at a certain temperature PCl5(g) ⇋ PCl3(g) + Cl2(g), the concentr...
Questions



Mathematics, 13.11.2020 21:50

Mathematics, 13.11.2020 21:50

Biology, 13.11.2020 21:50

Mathematics, 13.11.2020 21:50

Chemistry, 13.11.2020 21:50

Mathematics, 13.11.2020 21:50

Mathematics, 13.11.2020 21:50

Mathematics, 13.11.2020 21:50


Arts, 13.11.2020 21:50



Geography, 13.11.2020 21:50

Chemistry, 13.11.2020 21:50


Mathematics, 13.11.2020 21:50


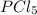 will be 0.9 M.
will be 0.9 M.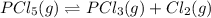
![[PCl_5]=0.40 M](/tpl/images/0534/1154/cb9c6.png)
![[PCl_3]=[Cl_2]=0.20 M](/tpl/images/0534/1154/9bc6a.png)
![K_c=\frac{[PCl_3][Cl_2]}{[PCl_5]}](/tpl/images/0534/1154/73fe0.png)
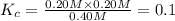
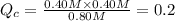
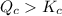
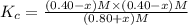
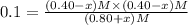
![[PCl_5]=(0.80+x) M=(0.80+0.1) M = 0.90](/tpl/images/0534/1154/dbad2.png)


