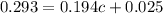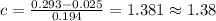A student's calibration curve for Blue #1 at 635 nm yields a straight line described by the equation y = 0.194 x + 0.025. If the measured absorbance for a solution of unknown concentration of Blue #1 is 0.293, what is the concentration of the Blue #1 solution? Provide your response to three digits after the decimal.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

Chemistry, 22.06.2019 15:30
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1
You know the right answer?
A student's calibration curve for Blue #1 at 635 nm yields a straight line described by the equation...
Questions

English, 22.04.2020 00:55






English, 22.04.2020 00:55

Mathematics, 22.04.2020 00:55

English, 22.04.2020 00:55







Mathematics, 22.04.2020 00:55

Mathematics, 22.04.2020 00:55

Mathematics, 22.04.2020 00:55


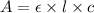
 = molar absorptivity coefficient
= molar absorptivity coefficient 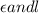 .
.