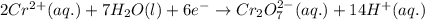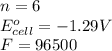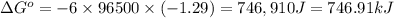Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1


Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
You know the right answer?
Calculate the standard reaction Gibbs free energy for the following cell reactions: (a) 2 Ce41(aq) 1...
Questions





Mathematics, 13.04.2021 03:40

Mathematics, 13.04.2021 03:40

Social Studies, 13.04.2021 03:40

History, 13.04.2021 03:40


Mathematics, 13.04.2021 03:40

Chemistry, 13.04.2021 03:40



Mathematics, 13.04.2021 03:40

Social Studies, 13.04.2021 03:40

History, 13.04.2021 03:40

Mathematics, 13.04.2021 03:40



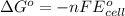 ............(1)
............(1)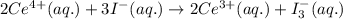
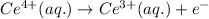 ( × 2)
( × 2)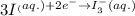
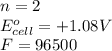
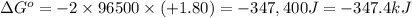
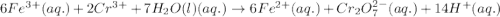
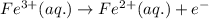 ( × 6)
( × 6)