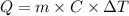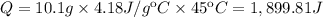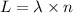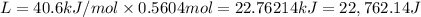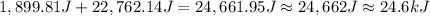Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 23.06.2019 00:00
This statement about matter and its behavior is best classified as a
Answers: 1
You know the right answer?
Calculate the energy (in kJ) required to heat 10.1 g of liquid water from 55 oC to 100 oC and change...
Questions

Mathematics, 06.04.2020 18:23

Mathematics, 06.04.2020 18:23






Mathematics, 06.04.2020 18:23

Mathematics, 06.04.2020 18:23



Biology, 06.04.2020 18:23

History, 06.04.2020 18:23

Social Studies, 06.04.2020 18:23


Chemistry, 06.04.2020 18:23

History, 06.04.2020 18:23