
Chemistry, 04.03.2020 01:57 isabelperez063
Excess aqueous copper(II) nitrate reacts with aqueous sodium sulfide to produce aqueous sodium nitrate and copper(II) sulfide as a precipitate. In this reaction 469 grams of copper(II) nitrate were combined with 156 grams of sodium sulfide to produce 272 grams of sodium nitrate.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1
You know the right answer?
Excess aqueous copper(II) nitrate reacts with aqueous sodium sulfide to produce aqueous sodium nitra...
Questions

Mathematics, 21.05.2021 18:50

History, 21.05.2021 18:50

Mathematics, 21.05.2021 18:50


Mathematics, 21.05.2021 18:50


Computers and Technology, 21.05.2021 18:50

Health, 21.05.2021 18:50

Mathematics, 21.05.2021 18:50

Mathematics, 21.05.2021 18:50

History, 21.05.2021 18:50



Mathematics, 21.05.2021 18:50


History, 21.05.2021 18:50

Mathematics, 21.05.2021 18:50


Chemistry, 21.05.2021 18:50

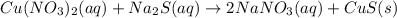
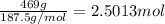
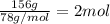
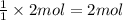 of copper (II) nitrate
of copper (II) nitrate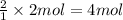 sodium nitrate
sodium nitrate 



