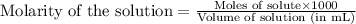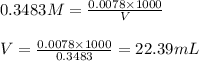Chemistry, 03.03.2020 05:59 egaitapierreval
Oxalic acid is a diprotic acid. If a solid material contains 53.66 percent of oxalic acid (H 2C 2O 4), by mass, then a 0.6543-g sample of that solid will require mL of 0.3483 M NaOH for neutralization. 11.19 97.78 28.59 1.119 22.39

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Which object forms when a supergiant runs out of fuel? a red giant a black hole a white dwarf a neutron star
Answers: 1


Chemistry, 23.06.2019 06:40
How many joules of heat are required to raise thetemperature of 750 g of water from 11.0 °c to 19.0 °c?
Answers: 1

Chemistry, 23.06.2019 08:00
Ineed this awnser fast select the correct answer. this chemical equation represents the burning of methane, but the equation is incomplete. what is the missing coefficient in both the reactants and the products? ch4 + → co2 + a. 0 b. 1c. 2d. 3 e. 4
Answers: 3
You know the right answer?
Oxalic acid is a diprotic acid. If a solid material contains 53.66 percent of oxalic acid (H 2C 2O 4...
Questions



Mathematics, 30.07.2019 17:30


Mathematics, 30.07.2019 17:30

Computers and Technology, 30.07.2019 17:30


Mathematics, 30.07.2019 17:30

Computers and Technology, 30.07.2019 17:30


Computers and Technology, 30.07.2019 17:30

Biology, 30.07.2019 17:30

History, 30.07.2019 17:30



Social Studies, 30.07.2019 17:30

History, 30.07.2019 17:30


Geography, 30.07.2019 17:30

Mathematics, 30.07.2019 17:30

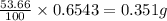
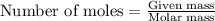
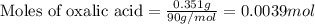
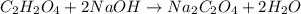
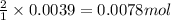 of NaOH
of NaOH