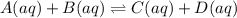A system at equilibrium has two aqueous products and two aqueous reactants. An additional amount of one of the products is added to the system. After the addition, which of the following will change?Select the correct answer below:a. the amount of the added productb. the amount of the other productc. the amounts of the reactantsd. all of the above

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1


Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1
You know the right answer?
A system at equilibrium has two aqueous products and two aqueous reactants. An additional amount of...
Questions


Mathematics, 14.09.2021 03:30

Mathematics, 14.09.2021 03:30

Chemistry, 14.09.2021 03:30


Mathematics, 14.09.2021 03:30




English, 14.09.2021 03:30

History, 14.09.2021 03:30

Mathematics, 14.09.2021 03:30

Mathematics, 14.09.2021 03:30

Mathematics, 14.09.2021 03:30


Mathematics, 14.09.2021 03:30

Mathematics, 14.09.2021 03:30


Mathematics, 14.09.2021 03:30

History, 14.09.2021 03:30