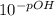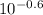A chemist must prepare 0.9 L of sodium hydroxide solution with a pH of 13.40 at 25°C. He will do this in three steps: Fill a 0.9 L volumetric flask about halfway with distilled water. Weigh out a small amount of solid sodium hydroxide and add it to the flask. Fill the flask to the mark with distilled water. Calculate the mass of sodium hydroxide that the chemist must weigh out in the second step.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1



You know the right answer?
A chemist must prepare 0.9 L of sodium hydroxide solution with a pH of 13.40 at 25°C. He will do thi...
Questions

Chemistry, 06.05.2020 19:57






History, 06.05.2020 19:57

Social Studies, 06.05.2020 19:57


Chemistry, 06.05.2020 19:57

Mathematics, 06.05.2020 19:57


English, 06.05.2020 19:57

Chemistry, 06.05.2020 19:57


Mathematics, 06.05.2020 19:57


Mathematics, 06.05.2020 19:57


Arts, 06.05.2020 19:57