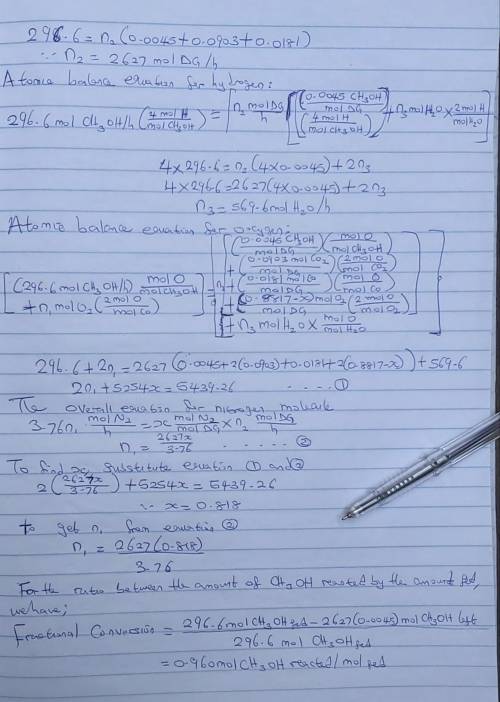
Liquid methanol is fed to a space heater at a rate of 12.0 L/h and burned with excess air. The product gas is analyzed and the following dry-basis mole percentages are determined: CH3OH = 0.45%, CO2 = 9.03%, and CO = 1.81%. (a) After drawing and labeling a flowchart, verify that the system has zero degrees of freedom. (b) Calculate the fractional conversion of methanol, the percentage excess air fed, and the mole fraction of water in the product gas. (c) Suppose the combustion products are released directly into a room. What potential problems do you see and what remedies can you suggest?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1
You know the right answer?
Liquid methanol is fed to a space heater at a rate of 12.0 L/h and burned with excess air. The produ...
Questions


Computers and Technology, 27.07.2019 11:00

English, 27.07.2019 11:00

History, 27.07.2019 11:00

History, 27.07.2019 11:00


Biology, 27.07.2019 11:00

Mathematics, 27.07.2019 11:00

Social Studies, 27.07.2019 11:00


Business, 27.07.2019 11:00


History, 27.07.2019 11:00

History, 27.07.2019 11:00


Biology, 27.07.2019 11:00


Biology, 27.07.2019 11:00