Chemistry, 03.03.2020 04:53 GhostElite6383
A 0.9679-g sample containing dimethylphthalate, (194.19 g/mol), and unreactive species was refluxed with 50.00 mL of 0.1215 M to hydrolyze the ester groups (this process is called saponification).
C6H4(COOCH3)2 + 2OH>> C6H4(COO)-2 + H2O
After the reaction was complete, the excess NaOH was back titrated with 32.25mL of 0.1251M HCl. Calculate the percentage of dimethylphthalate in the sample.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

Chemistry, 23.06.2019 01:30
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1
You know the right answer?
A 0.9679-g sample containing dimethylphthalate, (194.19 g/mol), and unreactive species was refluxed...
Questions

Mathematics, 08.10.2020 08:01

Biology, 08.10.2020 08:01

Mathematics, 08.10.2020 08:01

Social Studies, 08.10.2020 08:01


Mathematics, 08.10.2020 08:01

Physics, 08.10.2020 08:01

Law, 08.10.2020 08:01


Mathematics, 08.10.2020 08:01

Computers and Technology, 08.10.2020 08:01



Computers and Technology, 08.10.2020 08:01

Physics, 08.10.2020 08:01



Arts, 08.10.2020 08:01


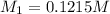
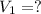

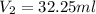
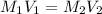
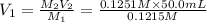
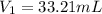
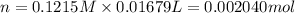

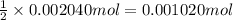 of dimethylphthalate
of dimethylphthalate